

ACIF

ALIF

PLIF
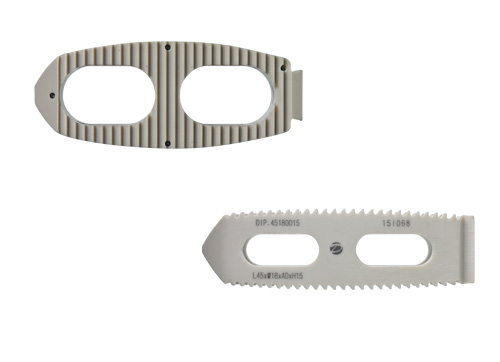
DLIF

TLIF-B

ALIF Cage
C-1
IVA ACIF cage
IVA ACIFcageis cervical interbody fusion devicesused to provide structural stability in skeletally mature individuals followingdiscectomy. IVA ACIF cageinserted through an anteriorcervical approach, and are available in various heights and geometricoptions to fit the anatomical needs of a wide variety of patients. Thesecages are filled with autogenous bone graft material. Protrusions onthe superior and inferior surfaces of each device grip the endplates of theadjacent vertebrae to resist expulsion.
IVA ACIF cage is interbody fusion devices intendedfor use in skeletally mature patients with degenerative disc disease (DDD)of the cervical spine (C3-T1) at one level. DDD is defined as discogenic painwith degeneration of the disc confirmed by history and radiographic studies.These patients should be skeletally mature and have had at least six (6)weeks of non-operative treatment.
IVA ACIF cage is filled with autogenous bone graft material. Thesedevices are intended to be used with supplemental fixation, such as Anterior Cervical Plate System
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Length Width | L11×W12 | L12×W14 | L14×W16 | L15×W18 |
| Angle | 0°, 7° | 0°, 7° | 0°, 7° | 0°, 7° |
| Height | H5~12 | H5~12 | H5~12 | H5~12 |


C-2
IVA cage(ALIF, PLIF, DLIF, TLIF) is lumbar interbody fusiondevices used to provide structural stability in skeletally mature individualsfollowing discectomy. Each of the IVA cage provides a differentshape to accommodate various surgical approaches to the lumbar spine(posterior, transforaminal, anterior, direct lateral). The devices are available in variousheights and geometric options to fit the anatomical needs of a widevariety of patients. These cages are filled with autogenous bonegraft material. Protrusions on the superior and inferior surfaces of eachdevice grip the endplates of the adjacent vertebrae to resist expulsion.
IVA cage(ALIF, PLIF, DLIF, TLIF) is interbody fusion devicesintended for use in patients with degenerative disc disease (DDD) at oneor two contiguous levels of the lumbosacral spine (L2-S1). DDD is definedas discogenic back pain with degeneration of the disc confirmed by historyand radiographic studies. These patients should be skeletally mature andhave had at least six (6) months of non-operative treatment. In addition,these patients may have up to Grade 1 spondylolisthesis or retrolisthesisat the involved level(s).
IVA cage is filled with autogenous bone graft material. Thesedevices are intended to be used with supplemental fixation, such as pedicle screw.
IVA ALIF cage
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Length Width | L20×W25 | L22×W29 | L24×W35 | L28×W39 |
| Angle | 0°, 8°, 15° | 0°, 8°, 15° | 0°, 8°, 15° | 0°, 8°, 15° |
| Height | H9~21 | H9~21 | H9~21 | H9~21 |


IVA PLIF cage
| 1 | 2 | 3 | |
|---|---|---|---|
| Length | L22 | L26 | L30 |
| Width | W8, W10 | W10, W12 | W10, W12 |
| Angle | 0°, 4°, 8° | 0°, 4°, 8° | 0°, 4°, 8° |
| Height | H7~17 | H7~17 | H7~17 |


IVA DLIF cage
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Length×Width | L40×W18 | L45×W18 | L50×W18 | L55×W18 | L60×W18 |
| Angle | 0°, 6°, 10° | 0°, 6°, 10° | 0°, 6°, 10° | 0°, 6°, 10° | 0°, 6°, 10° |
| Height | H7~17 | H7~17 | H7~17 | H7~17 | H7~17 |


IVA TLIF cage (Bullet)
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Length×Width | L40×W18 | L45×W18 | L50×W18 | L55×W18 | L60×W18 |
| Angle | 0°, 6°, 10° | 0°, 6°, 10° | 0°, 6°, 10° | 0°, 6°, 10° | 0°, 6°, 10° |
| Height | H7~17 | H7~17 | H7~17 | H7~17 | H7~17 |


IVA TLIF cage (Curved)
| 1 | 2 | 3 | |
|---|---|---|---|
| Length×Width | L28×W10 | L32×W10 | L36×W10 |
| Angle | 0°, 4°, 8° | 0°, 4°, 8° | 0°, 4°, 8° |
| Height | H7~16 | H7~16 | H7~16 |




